General
The HFH+MSU HS is a new entity created to support the collaboration between Henry Ford Health and Michigan State University. This entity has a Unique Entity Identifier (NH77YPDEGG84) and is nested under MSU.
The HFH+MSU HS is part of the FDP Expanded Clearinghouse but is not an FDP member institution. FDP Expanded Clearinghouse participants can use their own letter of intent when serving as subrecipients and should not be asked by other institutions to provide information that is readily available in the FDP Expanded Clearinghouse.
Proposals
The transition for proposal submission will be a phased approach. Currently, all direct or flow-through (subaward) NIH proposals with a lead PI from the MSU health colleges or HFH will be submitted under the HFH+MSU HS entity. Non-NIH proposals will continue to be submitted under the respective institutions, MSU or HFH.
Applications to other sponsors, including National Science Foundation, Department of Defense/CDMRP, and foundations, will continue to be submitted under MSU or HFH.
The types of submissions under HFH+MSU HS will expand as the collaboration progresses.
Health Colleges Research Services (HCRS) will automatically set up proposals under HFH+MSU HS or MSU as outlined above based on sponsor and lead unit/college. Henry Ford Health (HFH) will no longer be included as a subaward in direct NIH proposal submissions under HFH+MSU HS. Instead, we will budget and include HFH involvement as a direct cost. HCRS will communicate with HFH to obtain salary and project cost information for collaborators at HFH.
The HCRS Proposal Intake Form will now includes a section to indicate HFH involvement in a proposal. Please provide contact information for HFH collaborators or administrators, if known. It is imperative HCRS receive at least 8 weeks’ notice of intent to submit so we have time to work on the collaborative proposal process.
All PDs will route for approval, and all MSU key personnel will need to submit a project-based disclosure. All other approvals, such as cost share or PI exception approval, will follow standard MSU procedures.
In an HFH+MSU HS submission, the applicant organization will now show as Henry Ford Health+Michigan State University Health Sciences.
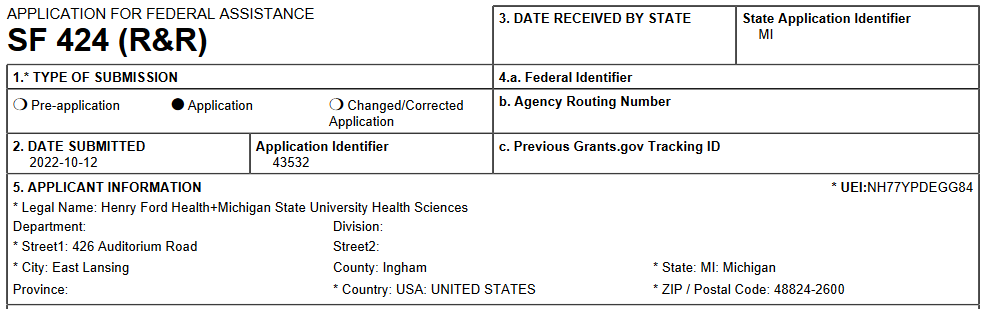
Henry Ford Health + Michigan State University Health Sciences (HFH+MSU HS) is a unique partnership between Henry Ford Health System and Michigan State University. The partnership is focused on research, education and care, and is initiating programs in crucial areas affecting health and wellness including health equity, cancer, population health and neuroscience. At this time over 100 researchers from Henry Ford Health have been appointed at Michigan State University, creating new synergies for collaboration and innovative research. Additional faculty will be added in the future to include clinician researchers.
Please use the following template language: "Henry Ford Health + Michigan State University Health Sciences (HFH+MSU HS) is a partnership between Henry Ford Health System and Michigan State University. The Unique Entity Identifier for the partnership rolls up to Michigan State University (MSU) and is included in MSU’s F&A rate agreement. MSU’s Facilities and Administrative (F&A) research rate is 57% for the period from July 1, 2023 to June 30, 2027. Modified Total Direct Costs (MTDC) is the base to which the F&A rate is applied. MTDC consists of all direct salaries and wages, applicable fringe benefits, materials and supplies, services, travel and up to the first $25,000 of each subaward. MTDC excludes equipment, capital expenditures, charges for patient care, rental costs, tuition remission, scholarships and fellowships, participant support costs and the portion of each subaward in excess of $25,000. This agreement was negotiated through the Department of Health and Human Services, Olulola Oluborode, telephone 214-767-3261."
NIH resubmissions and renewals will be processed under HFH+MSU HS. Research administrators and Grant Managers will include a “change of grantee” section in the proposal indicating Michigan State University or Henry Ford Health as the previous applicant. We will include a cover letter with the proposal indicating the relinquishment of the resubmission/renewal to HFH+MSU HS.
Resubmissions and renewals to other sponsors will be processed under MSU or HFH at this time. Please reference Proposals Q1 for details about the phased transition.
Submissions under HFH+MSU HS will appear under the new organization. PIs must select the correct institution in eRA Commons to view a submission. If you do not see a recent submission in eRA Commons, you may not be viewing proposals under the correct institution. For instructions on how to change the affiliated institution in eRA Commons, see: Changing the Displayed Affiliated Institution.
Yes, please use the HFH+MSU HS commitment letter when HFH+MSU HS is named as the subrecipient.
The HFH+MSU HS Letter of Intent can be used by subrecipients who are part of the FDP Expanded Clearinghouse when HFH+MSU HS is the direct receipient of federal funds.
When naming another institution as a subrecipient who is not part of the FDP Expanded Clearinghouse, please use the subrecipient commitment form
Awards
Current MSU awards will remain with MSU. Some HFH awards will be transferred in the future.
For MSU Faculty and Staff
All faculty with a lead/primary appointment in the Colleges of Human Medicine, Osteopathic Medicine, or Nursing will be considered a member of the HFH+MSU HS. Please refer to the memo from the VP for Research and Innovation describing impacts on the proposal process for MSU investigators.
No, faculty outside of the health colleges are not considered members of the HFH+MSU HS at this time and will continue to submit proposals under MSU. They may be a collaborator (Co-I, Key Personnel) on a proposal that is submitted through HFH+MSU HS.
Newly proposed NIH subawards will be processed under HFH+MSU HS for faculty in the health colleges. All other subawards will be processed under MSU. We will transition to submitting additional types of subawards under the HFH+MSU HS entity per the phased approach described in Proposals Q1.
Under this new arrangement, attribution will flow to your college and department as it does now. F&A costs recovery will be distributed according to the standard practice and methodology for distribution of funds to colleges and departments.
For HFH Faculty and Staff
HFH faculty named as a PI, Multi-PI, or Co-Investigator must have an MSU appointment. Please refer to the HFH Investigator Eligibility table for more details. Contact Kathy Huber, khuber1@hfhs.org, for assistance with an appointment.
Yes, both direct and flow-through (subaward) NIH proposals will be processed through HFH+MSU HS. Please see the HFH+MSU HS commitment letter for use in flow-through applications.
Most of this information is either automatically populated or available by user selection of search fields in Kuali Research. For NIH budget forms, the HFH+MSU HS UEI is NH77YPDEGG84.
 HFH employees with an MSU appointment (appointment is required for PI’s/Co-I’s/Multi PI’s and highly recommended for all other key person roles):
HFH employees with an MSU appointment (appointment is required for PI’s/Co-I’s/Multi PI’s and highly recommended for all other key person roles):
- Project-based COI disclosures will be done in MSU’s COI Disclosure system. The disclosure is not created until the PD is routed. Once routed, you can check the status of a project-based disclosure from the COI Disclosure suboption (screenshot right). The job aid for approving a PD and submitting a COI is available on the MSU website.
- Annual Disclosures will be done in HFH system. HFH employees must have an up-to-date annual disclosure prior to proposal submission. The HFH Central Office will provide MSU the status of annual disclosures on behalf of HFH employees.
HFH employees without an MSU appointment (only allowed for Key Persons that do not have the PI/Co-I/Multi PI role):
- Project based COI disclosures will be done using MSU’s paper COI form. Disclosures must be completed prior to submission and included in proposal packet.
- Annual Disclosures will be done in HFH system. HFH employees must have an up-to-date annual disclosure prior to submission. The HFH Central Office will provide MSU the status of annual disclosures on behalf of HFH employees.
Subawardee key persons:
- Review answer to the COI question in the Subrecipient Commitment Form:
- If yes, the subrecipient has a PHS compliant policy, no further action is required.
- If no, the subrecipients does NOT have a PHS compliant policy, the subrecipient must follow MSU’s policy, and key personnel at the site must complete MSU’s paper COI form. All disclosures must be completed prior to submission and included in proposal packet.
Outside consultants:
- Consultants must follow MSU’s policy and need to complete MSU’s paper COI form. Disclosures must be completed prior to submission and included in proposal packet.
Credit allocation is required before routing a PD. The total F&A allocation must equal 100%. Please use this template to assist you in calculating the credit for each investigator. Directions are included as comments in the header row of the table.
Yes, please reference this NIH R series checklist for HFH lead proposals.
NOTE: This checklist is meant for use when submitting a proposal directly NIH. If you are working on a PD for a subaward proposal, instructions are available here: https://osp.msu.edu/PL/Portal/820/PreparingaSummaryProposal
KR Training Materials: https://cga.msu.edu/PL/Portal/787/KualiResearchKRTraining
Suggested Email Templates for Communication with OSP: https://hcrs.msu.edu/images/documents/Email_Templates_OSP.docx
Step-by-Step KR Instructions: https://cga.msu.edu/PL/Portal/3644/PreparinganNIHProposalinKualiResearch(KR)
Avoiding Common NIH Submission Errors: https://grants.nih.gov/grants/how-to-apply-application-guide/learn-how-we-check-your-application-for-completeness/avoiding-common-errors.htm
NIH Application Guide:https://grants.nih.gov/grants/how-to-apply-application-guide/forms-g/general/g.100-how-to-use-the-application-instructions.htm
NIH Page Limits: https://grants.nih.gov/grants/how-to-apply-application-guide/format-and-write/page-limits.htm
Additional assistance with Human Subjects determinations:
- Decision Tool: Am I Doing Human Subjects Research? – Decision tree to help determine if you’re doing human subjects research
- Human Subjects Research Infographic – One page infographic with examples of human subjects research
- Exempt Human Subjects Research Infographic – One page infographic explaining the human subject exemptions
- PHS Human Subjects and Clinical Trials Information – provides NIH guidance on how to complete the fields within the PHS Human Subjects and Clinical Trials Information Form, including the Study Record and Planned/Cumulative enrollment form.
This page will be updated as the collaboration expands.